
Plant Cell Study Adds to Protein Trafficking Dogma
Quick Summary
- A new study adds nuance to the idea that proteins must be unfolded to cross cellular membranes
- Using green plants, researchers found the protein DHFR could cross the chloroplast membrane unfolded
- The findings could help researchers advance biotechnologies beneficial for agriculture
There’s a prevailing dogma in the field of cell biology. When it comes to transmembrane protein trafficking—the act of molecules crossing cellular membranes—proteins need to be unfolded to cross, otherwise they’re too bulky to fit through membrane pores.
“Proteins are polymers of amino acids linked end to end, like beads on a string, and they fold into compact structures and that’s the only time they can do what they’re supposed to do,” said Professor Steven Theg, Department of Plant Biology. “But to transport a protein across a membrane, you generally have to unfold it completely and send it across bead by bead.”
A new study, published by Theg and his colleagues in The Plant Cell, reexamines how protein trafficking occurs in the chloroplasts of green plants. The researchers found that surprisingly large proteins don’t require unfolding to cross the chloroplast membrane. They can do so while folded.
Since much of the metabolic activity in plant cells occurs in chloroplasts, understanding the protein trafficking process could help advance biotechnologies beneficial for agriculture. For example, to create plants resistant to the herbicide glyphosate, researchers targeted a specific protein to chloroplasts to give crops such resistance.
“If you want to affect some metabolic change in plant cells, you’re often targeting proteins to chloroplasts,” Theg said.

Breaching the barrier
In plant cells, chloroplasts rely heavily on imported proteins to achieve functions like photosynthesis. According to Theg, around 95 percent of all chloroplast proteins are imported from a plant cell’s cytoplasm. That’s a lot of unfolding and refolding.
Theg and colleagues in his lab started testing the hypothesis behind the protein trafficking dogma about five years ago. They used a protein called DHFR, which has long been used by researchers interested in protein trafficking. DHFR can be stabilized in its structure by binding tightly to an inhibitor called methotrexate. This prevents DHFR import into other organelles like mitochondria and the endoplasmic reticulum.
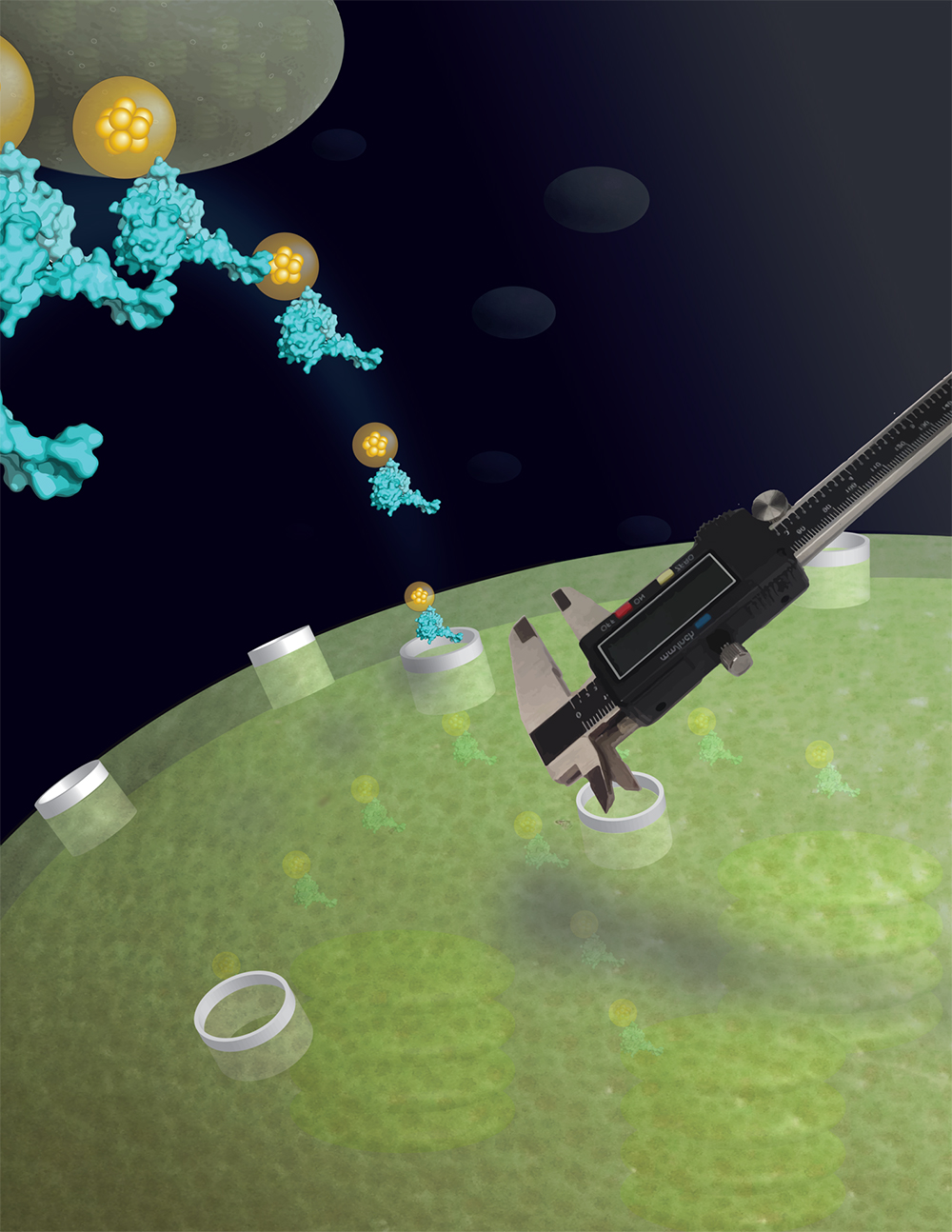
This finding initially led to the idea that proteins must unfold to cross membranes, and it was noted that methotrexate did not block DHFR import into chloroplasts. At the time, the researchers hypothesized that DHFR sheds the inhibitor and unfolds before crossing the chloroplast membrane. Once inside, the protein refolds, allowing it to rebind the inhibitor that had entered the chloroplasts on its own.
Theg and colleagues challenged this hypothesis using a fluorescent version of methotrexate that cannot independently cross chloroplast membranes and monitored its effect on DHFR’s import into chloroplasts.
“If DHFR brings the methotrexate into the chloroplast that means it remained folded because it only binds the inhibitor as a folded protein,” explained Theg. Sure enough, methotrexate remained bound to DHFR throughout the translocation process, proving that the protein could pass through both the outer and inner chloroplast envelope membranes in its folded state.
“The ability to import folded proteins across a double-membrane barrier is unique to chloroplasts,” said Iniyan Ganesan, a study author and plant biology Ph.D. student. “This changes the way we view protein translocation in general because organelles typically require unfolded protein import substrates.”
To confirm their findings, the researchers performed experiments in which they replaced DHFR with gold particles, which are completely rigid in structure and can’t be unfolded. Like DHFR, the gold particle passed through the chloroplast membrane without a problem.
Using fixed-diameter particles, the researchers also determined that the pore size of the chloroplast membrane was greater than 26 angstroms, a unit of measure equal to one hundred-millionth of a centimeter. This is large enough to allow DHFR to pass through the membrane folded. It’s also larger than the protein transport pores of mitochondria and bacteria.

Questioning dogma
Theg emphasized that the new study doesn’t upend the dogma of protein trafficking. Rather, it adds nuance to the process.
“Science often advances this way, where we have an idea that’s been so helpful for the last 30 years and has explained so many things but is not the whole story,” Theg said. “The surprise was that in this particular case, we found that rather large proteins don’t have to unfold to traverse chloroplast membranes.”
“I think proteins probably do unfold to cross membranes much of the time,” he added. “It’s not that the dogma is wrong. It’s just that the dogma does not paint the complete picture.”
The research was supported by the National Science Foundation and the Department of Energy.
