Fly Brain Holds Secrets of Body Temperature and Sleep
Neural pathways common to humans and flies provide an opportunity to advance medicine
The mind of a fruit fly encompasses 125,000 nerve cells, squeezed into the space of a poppy seed. At first glance, the fly brain looks nothing like a human brain. But many of the underlying neural circuits are surprisingly similar.

Fumika Hamada, a professor of neurobiology, physiology, and behavior, is using fruit flies to study a critical but oft-overlooked brain function: the regulation of our body temperature in a consistent daily rhythm.
“All animals have body temperature rhythms, from flies to fish and humans,” said Hamada. Body temperature influences countless body functions including sleep, blood pressure, and metabolism. “The brain sets the pace of this daily rhythm and uses body temperature to synchronize all other organs.”
Hamada is identifying the molecules and brain cells that do this in fruit flies (Drosophila melanogaster). Her work could lead to advances in human medicine, including new treatments for sleeping disorders, and perhaps even new ways to prevent the onset of metabolic syndrome — the constellation of weight gain, diabetes, high blood pressure, and immune dysfunction that undermines the health of millions of Americans.
Flies and humans have similar rhythms
Average human body temperature is around 37°C (98.6°F), but our temperature actually varies by a small but meaningful amount over the course of 24 hours. It falls by about one degree at night and rises throughout the day.
“That evening reduction in body temperature is a cue for the onset of sleep,” says Hamada.
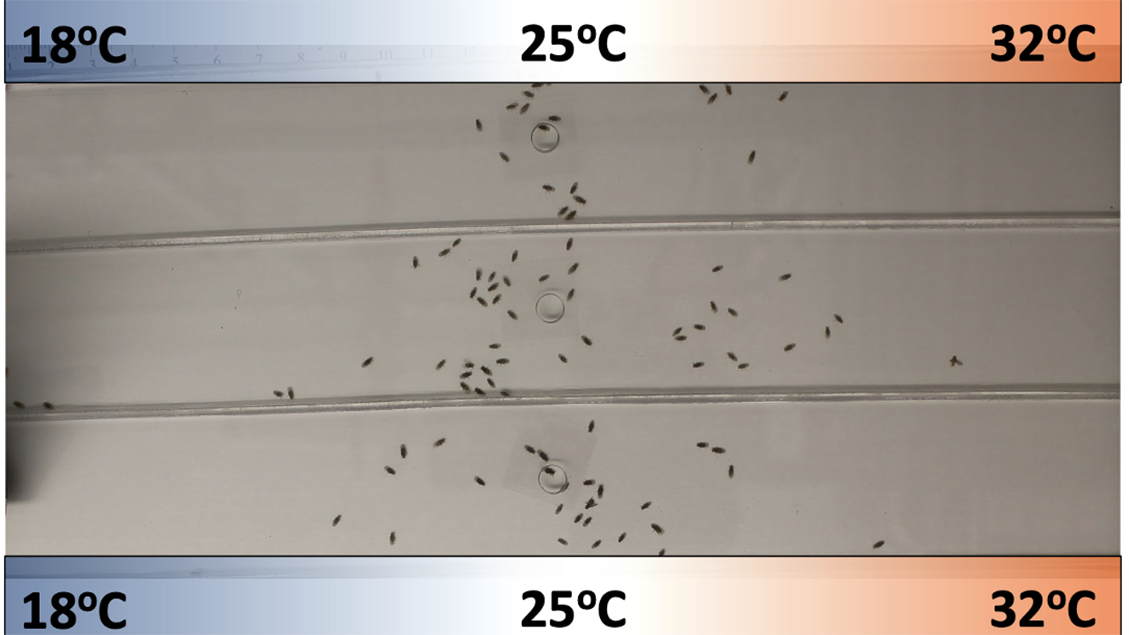
She has found that the body temperature of flies follows a similar rhythm. Because flies are cold-blooded, they can’t regulate their body temperature metabolically as mammals do. Instead, they regulate it behaviorally by perching in places that match the temperature they prefer at a given moment. If Hamada puts flies in a long chamber where the temperature ranges from 18°C at one end to 32°C at the other, most of them hang out in the middle, where the temperature is around 25°C. But at night the flies like it a little cooler and move to where the temperature is closer to 23.5°C.

“For a long time, people knew little about the mechanism of body temperature rhythm in insects or mammals,” says Hamada. But when she began studying it in flies, she made two major discoveries.
First, she found that in flies this daily rhythm is controlled by a simple network of brain cells, called dorsal neurons. Three sets of these neurons are the key regulators of temperature preference rhythm, and without them, flies lose their rhythm.
Second, she found that some of these neurons require a specific protein, called DH31R, to maintain the temperature rhythm. Of special note is that fruit fly DH31R is almost identical to a protein in mice, humans, and other mammals, called calcitonin receptor. In another experiment, to test if the functions of these two proteins are similar, Hamada found that the calcitonin receptor appears to regulate temperature rhythms in mice. This further illustrates the parallels of molecular pathways between flies and mammals.
For Hamada, these discoveries highlight another important set of questions she hopes to investigate. “Because body temperature is related to sleep, we think this could help us learn more about the regulation of sleep in both flies and mammals,” she says.
Links to eating and metabolism
Hamada is also mapping out other important similarities in the way that humans and flies regulate temperature. It is known, for example, that prolonged starvation in humans and other mammals causes a slight drop in body temperature, which may conserve calories by slowing metabolism.
She has found that hungry flies prefer a slightly cooler temperature in much the same way that mammals do. Once the flies have eaten, they go back to preferring the normal warmer temperature. And she has discovered that in flies, this interplay between hunger, satiety, and body temperature is driven by a molecule that is nearly identical to the human hormone insulin. This discovery is interesting, because in humans, insulin plays a critical role in regulating blood sugar levels – and the disruption of this function leads to diabetes.
Most recently, in 2024, Hamada discovered that even the mere taste of sweet food can trigger a fly’s temperature preference to increase slightly — even if that taste comes from an artificial sweetener. Humans and other mammals have a similar response to the initial taste of food.
Because of these strong similarities, Hamada believes that her experiments could lay the groundwork for improving medical care for humans. Figuring out how changes in temperature prepare the body for sleep could identify key proteins and neurons. Researchers could then develop new drugs targeting them to treat sleeping disorders.
Deciphering the connections between eating, insulin, body temperature, and sleep could also yield new treatments for other common ailments.

Scientists increasingly recognize that the chronic sleep loss experienced by many Americans can trigger appetite swings, weight gain and abnormal insulin signaling. This leads to poor regulation of blood sugar, and eventually diabetes and high blood pressure. Chronic sleep loss can also impact the immune system, leading to inflammation that increases the risk of heart attacks. Hamada believes that studies in flies could identify the key molecules and nerve cells that are behind these links – opening the door for human studies and improved medical care.
The long-term goal is to address a slow-moving epidemic and reduce the burden of chronic disease in Americans and people around the world. “We can work more quickly in flies,” says Hamada, “because we have so many research tools that allow us to alter gene function precisely, in specific cells in the fly brain.”
Hamada’s work is funded by the National Institutes of Health. Her work utilizes research core facilities at UC Davis, including the West Coast Metabolomics Center and the DataLab.

Media Resources
- Hamada Lab
- Taste triggers a homeostatic temperature control in hungry flies (eLife 2024)
- Dorsal clock networks drive temperatures preference rhythms in Drosophila (Cell Reports 2022)
- Feeding-state dependent modulation of temperature preference requires insulin signaling in Drosophila warm-sensing neurons (Current Biology 2018)
- Calcitonin receptors are ancient modulators for rhythms of preferential temperature in insects and body temperature in mammals (Genes & Development 2018)
- Circadian rhythm of temperature preference and its neural control in Drosophila (Current Biology 2012)
- Douglas Fox is a freelance science writer based in the Bay Area.
