Gatekeeper of the Cellular Highway: Study Reveals Novel Behaviors of the Alzheimer’s Disease Protein Tau
Quick Summary
- Neurofibrillary tangles called "tau tangles" are characteristic of those with Alzheimer's disease or traumatic brain injury
- The natural physiological function of tau in healthy brains remains a mystery
- In a new study, UC Davis researchers found tau molecules congregate together in a reversible way that appears to be distinct from the tau tangles seen in neurodegenerative diseases
In the brains of those with Alzheimer’s disease, traumatic brain injury and other neurodegenerative disorders, insoluble fibers composed of a protein called tau build up inside of neurons, eventually creating a tangled mess characteristic of these diseases.
“These tangles grow and grow and grow inside of the cells and at some point that gums up the works so much that the cell just dies,” said Assistant Professor Richard McKenney, Department of Molecular and Cellular Biology. “But (after death) the protein plaques are left behind and can possibly spread to other cells in the brain.”
Tau tangles (or ‘neurofibrillary tangles’) have become a litmus test for Alzheimer’s disease. But are they the cause of it or the result? The physiological function of tau inside of neurons remains a mystery, hampering an understanding of what tau normally does, and what goes wrong when tau tangles form inside of neurons.
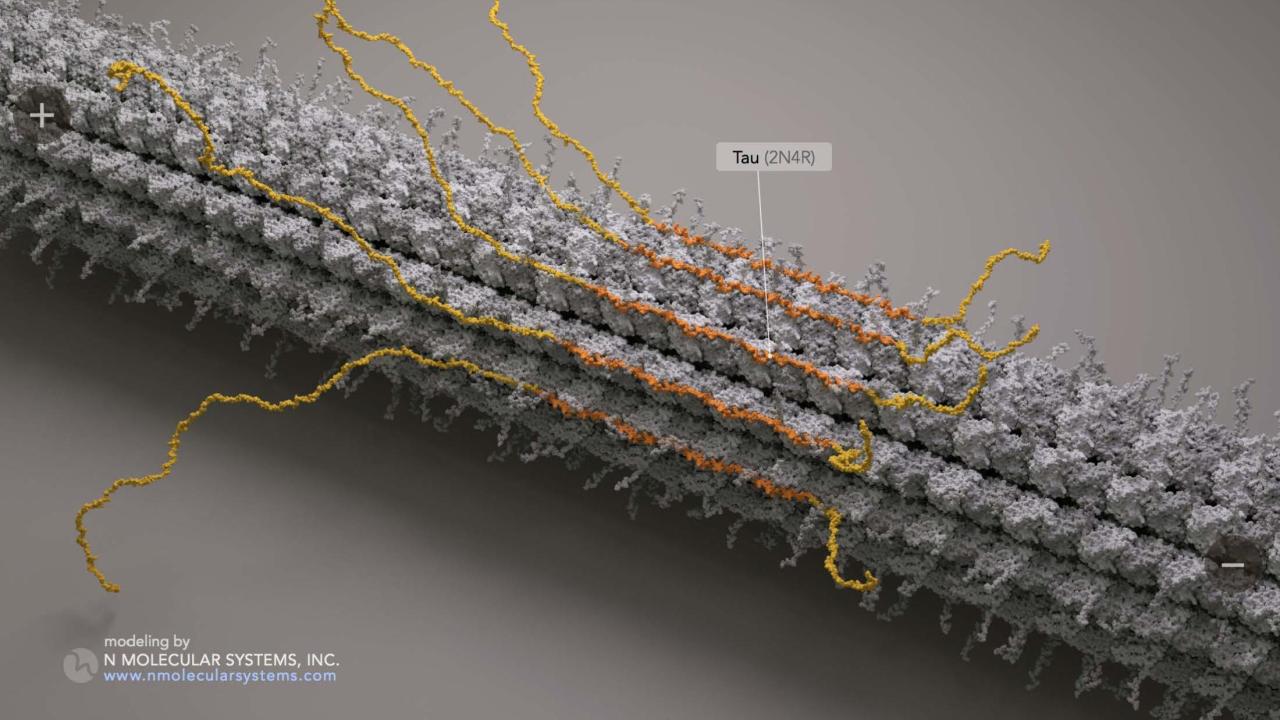
In a new study appearing in Nature Cell Biology, McKenney, Assistant Professor Kassandra Ori-McKenney and their colleagues found that tau molecules can congregate together in a novel, reversible way, which appears to be distinct from the irreversible tangle formation observed in neurodegenerative diseases. Once they come together, groups of tau molecules act like gatekeepers along the cell’s microtubules, the highways of the cell’s molecular transport system.
“Using a technique that allows us to visualize the behavior of single tau molecules, we directly observed that tau can self-associate even in the absence of a disease state,” said McKenney. “We’re proposing that self-association of tau molecules is actually a normal thing that can somehow go wrong and cause these neurodegenerative diseases.”
The new research upends the dogma that tau typically exists as a single molecule along the cell’s highways and that tau self-association is only present in diseased brain cells.
“There must be a physiological function for this protein, even though most studies focus on it in the diseased state,” said Ori-McKenney. “The problem with that is if you only focus on the disease states there’s only so far you can go. In order to really understand how it goes awry, you need to understand its normal function.”

A molecular traffic light
Tau belongs to a large group of proteins called microtubule-associated proteins (MAPs), which bind to intracellular microtubules and can affect the transport functions of the motor proteins dynein and kinesin. Using a technique called single-molecule microscopy, Ori-McKenney, McKenney and their team directly observed interactions between purified tau molecules and purified microtubules outside of the cell environment.
“We immediately saw that they were behaving differently than other types of MAPs that we had looked at in the lab,” said McKenney.
In the experiments, the tau molecules congregated together in specific regions along the microtubules, which McKenney and Ori-McKenney refer to as “condensates.” Compartmentalizing the microtubule, these condensates, according to the researchers, were formed through the dynamic self-association of tau molecules. The researchers believe this novel behavior of tau was driven by a process known as phase separation.
“The most simple way to think about this is oil and water,” said McKenney. “When you mix oil and water, the oil separates itself out from the water. This is due to the self-association of the oil molecules with each other, akin to the self-association of tau molecules that we observe in our experiments.”
To uncover the function of these tau condensates, the team tested their effects on molecular motor proteins and another protein called spastin, a severing enzyme that cuts and remodels microtubules within cells. The researchers found that the condensates acted like a selective molecular sieve, allowing some motor proteins, but not others, to pass through, fully protecting the microtubule from spastin’s severing activity.
“By forming a selectively permeable sheath around the microtubule, the condensate is saying to these other molecules, ‘Okay, you can act there and there, but you can’t act here,’” said McKenney.
The condensates, the researchers found, acted like directional traffic lights for the motor proteins dynein and kinesin.
“I think that tau is a really excellent candidate for being one of the major traffic lights within the axon of the neuron in terms of its direction of motor transport and the other enzymes that act on the microtubule,” said Ori-McKenney.
Protein talk on the patio
Discovers like this drive the curiosity and after-hours discussions between Ori-McKenney and McKenney. Now that they understand tau naturally forms these condensates, Ori-McKenney and McKenney have many new questions. What leads to the unwieldy tau tangles present in neurodegenerative diseases? Are they a byproduct of some kind of dysfunction of tau’s condensation? An intriguing possibility since self-association of tau molecules underlies both processes.
“This paper, like much of our work, just kind of sprung out of our collective interest in the biology of the cell’s cytoskeleton,” said Ori-McKenney. “We sit on our patio at night and discuss what are the most interesting directions we can take in order to understand this protein and others in more depth.”
The researchers hope to partner with the UC Davis Alzheimer’s Disease Center in an effort to obtain patient brain samples and expand their research on tau behavior to include tau in a genuinely pathological-state. The research was carried out by Biochemistry, Molecular, Cellular and Developmental Biology graduate students Ruensern Tan, with help from Tracy Tan, and also undergraduate Aileen Lam, who is the recipient of the 2019 Hanson Family Undergraduate Research Publication Award. The research was funded by grants from the National Institutes of Health and the Pew Foundation.

