New Research Identifies Protein Integral to Sperm Development and Male Fertility
A little-known protein helps safeguard genomic stability while chromosomes are cut and spliced back together
Early in the development of sperm, a strange event happens: the X and Y chromosomes condense into tight packages and are sequestered away from the other 44 human chromosomes. If any part of this process goes awry, the cells cannot mature into sperm. Researchers in the College of Biological Sciences have now identified an important link in this process — a little-known protein called ATF7IP2.
“This could be a critical factor for ensuring male fertility,” says Satoshi Namekawa, a professor of Microbiology and Molecular Genetics, whose team contributed to the new findings. Their results, published Feb. 21 in Genes & Development, could help elucidate the causes of male infertility.
A dangerous moment for DNA
The discovery sheds light on a pivotal moment in the production of sperm that is necessary for the health of our species — but also potentially dangerous.
The cells that give rise to sperm contain 46 chromosomes — two copies each of chromosomes 1 through 22, plus one of each sex chromosome, X and Y. The sperm will eventually carry only half a set — 23 chromosomes. But before they are divided up, the 22 sets of homologous chromosomes pair up, and segments of DNA are swapped between each pair. This recombination shuffles the genetic deck — ensuring that the next generation of humans will have diverse genes that determine disease resistance and many other traits. But recombination carries risks.
The DNA must be cut and rejoined dozens of times without a single error. If the wrong chromosomes are paired up, the wrong cuts are made, or the wrong ends are rejoined, the resulting embryo may fail to develop, or the offspring may end up missing genes, or with extra copies — triggering genetic diseases.

Namekawa and his team have spent years studying the way that germline cells (which produce sperm and egg cells) prevent this from happening. They and others have found that a constellation of proteins called the DNA damage response (DDR) guides the process. When a person is exposed to radiation, chemicals, or anything else that breaks DNA, the DDR ensures that the resulting loose ends are correctly re-attached. DDR plays a similar role in recombination, making sure that chromosomes only pair up with their twins — and that cuts are rejoined.
But unlike other pairs of chromosomes, X and Y are actually different from one another. If they swap parts, this could damage the genome, says Kris Alavattam, a former Ph.D. student at Namekawa’s lab, now at the Fred Hutchinson Cancer Center in Seattle. “When X and Y are unable to match up, the DDR causes them to come together into their own compartment, away from the other chromosomes,” he says.

This happens when an enzyme called SETDB1 modifies the protein spools that the X and Y chromosomes’ DNA is wrapped around, causing it to coagulate into a dense structure called heterochromatin. The resulting inactivation prevents recombination and silences the X’s and Y’s genes at the correct time to prevent them from interfering with the process of divvying up chromosomes into sperm cells.
A likely suspect
In 2016, Alavattam and Namekawa looked for a molecular link between DDR and the inactivation of the X and Y chromosomes. It could have been any one of hundreds of proteins, but Alavattam and Namekawa noticed that one little-known protein, called ATF7IP2, was abundant in sperm-forming cells undergoing recombination — and was virtually absent from all other tissues. They also knew that the ATF7IP2 protein sometimes attaches to the SETDB1 enzyme.
“It suggested that ATF7IP2 might regulate SETDB1 and recruit it to the X and Y chromosomes,” says Alavattam.
Alavattam did experiments to answer this question until graduating with his Ph.D. in 2020. A new Ph.D. student, Jasmine Esparza, then joined Namekawa’s lab in 2021 and continued the experiments. Also collaborating on the experiments were Ryuki Shimada, Ph.D. in the laboratory of Kei-ichiro Ishiguro, Ph.D. at Kumamoto University, in Japan.
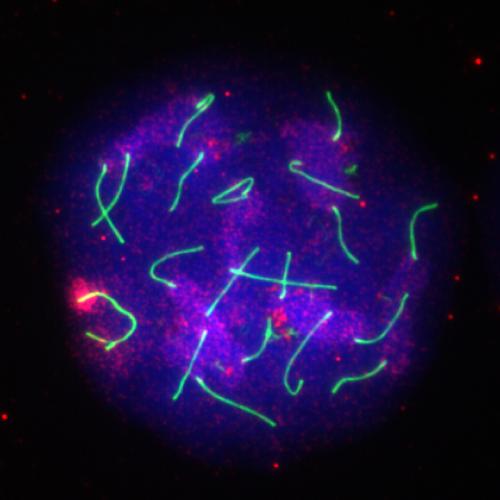
In the newly published work, Alavattam, Namekawa, and Esparza found that male mice with the ATF7IP2 gene disabled are healthy but infertile, with no sperm. In the cells that would normally become sperm, the SETDB1 enzyme doesn’t modify the X and Y chromosomes, and these two chromosomes aren’t compacted into heterochromatin. Together, these results suggest that ATF7IP2 plays an indispensable role in the development of sperm and is necessary for male fertility.
Esparza and Namekawa, together with project scientist Mengwen Hu, found that ATF7IP2 also plays other roles in sperm development. In addition to targeting X and Y, it causes SETDB1 to silence genetic parasites called retroelements, which are scattered across all of the chromosomes and can cause genomic errors.
They also found that ATF7IP2 plays another, seemingly opposite role: it activates certain genes on the other, non-sex chromosomes, which are important in recombination and sorting of chromosomes into sperm cells.
“It’s very surprising” to see that ATF7IP2 has such diverse functions, says Namekawa.
Studying this protein could reveal some causes of male infertility. Namekawa and Esparza are already looking into new questions — hoping to find out how ATF7IP2 activates some parts of the other 44 chromosomes, even as it inactivates X and Y. For that job, it might link up with proteins other than SETDB1. “We’ve identified a really important pathway,” says Namekawa. “We intend to follow it to see where it leads.”
Additional authors on the paper include: at UC Davis, Yasuhisa Munakata, Kai Otsuka, Yuka Kitamura, and Yu-Han Yeh; at UC Davis and Kumamoto University, Hironori Abe; at Cincinnati Children’s Hospital Medical Center, Anna R. Kohrs; at Kumamoto University, Saori Yoshimura; at Cincinnati Children’s Hospital Medical Center and University of Cincinnati College of Medicine, Yueh-Chiang Hu and Paul R. Andreassen; at University of Tokyo, Jihye Kim. Funding was provided by grants from the National Institutes of Health.
Media Resources
- Douglas Fox is a freelance science writer based in the Bay Area.
- ATF7IP2 / MCAF2 directs H3K9 methylation and meiotic gene regulation in the male germline (Genes & Development 2024)
- Active DNA damage response signaling initiates and maintains meiotic sex chromosome inactivation (Nature Communications 2022)
- The Initiation of Meiotic Sex Chromosome Inactivation Sequesters DNA Damage Signaling from Autosomes in Mouse Spermatogenesis (Current Biology 2020)
